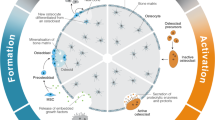
Abstract
Purpose of Review
Bone mineral density and systemic factors are used to assess skeletal health in astronauts. Yet, even in a general population, these measures fail to accurately predict when any individual will fracture. This review considers how long-duration human spaceflight requires evaluation of additional bone structural and material quality measures that contribute to microgravity-induced skeletal fragility.
Recent Findings
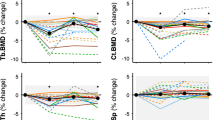
In both humans and small animal models following spaceflight, bone mass is compromised via reduced bone formation and elevated resorption levels. Concurrently, bone structural quality (e.g., trabecular microarchitecture) is diminished and the quality of bone material is reduced via impaired tissue mineralization, maturation, and maintenance (e.g., mediated by osteocytes).
Summary
Bone structural and material quality are both affected by microgravity and may, together, jeopardize astronaut operational readiness and lead to increased fracture risk upon return to gravitational loading. Future studies need to directly evaluate how bone quality combines with diminished bone mass to influence bone strength and toughness (e.g., resistance to fracture). Bone quality assessment promises to identify novel biomarkers and therapeutic targets.


Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Hackney K, English K, Hackney KJ, English KL. Protein and essential amino acids to protect musculoskeletal health during spaceflight: evidence of a paradox? Life. 2014;4:295–317.
Michel EL, Johnston RS, Dietlein LF. Biomedical results of the Skylab Program. Life Sci Space Res. 1976;14:3–18.
Johnston RS, Dietlein LF. In: Johnst RS, Dietlein LF, editors. 491 pages Biomedical results from Skylab. NASA SP-377. Biomed. Results from Skylab. NASA SP-377, vol. 377. Washington, D.C: NASA; 1977.
NASA Human Research Program. Integrated Research Plan. HRP 47065 Rev J PCN 1; 2019.
Human Research Roadmap. NASA Human Resarch Program Website; 2019. Available at: humanresearchroadmap.nasa.gov. Accessed 5 June 2019.
Sibonga JD. Spaceflight-induced bone loss: is there an osteoporosis risk? Curr Osteoporos Rep. 2013;11:92–8.
Deymier AC, Schwartz AG, Cai Z, Daulton TL, Pasteris JD, Genin GM, et al. The multiscale structural and mechanical effects of mouse supraspinatus muscle unloading on the mature enthesis. Acta Biomater. 2019;83:302–13.
Cohen HS, Kimball KT, Mulavara AP, Bloomberg JJ, Paloski WH. Posturography and locomotor tests of dynamic balance after long-duration spaceflight. J Vestib Res. 2012;22:191–6.
Reschke MF, Clément G. Vestibular and sensorimotor dysfunction during space flight. Curr Pathobiol Rep. 2018;6:177–83.
Ozdemir RA, Goel R, Reschke MF, Wood SJ, Paloski WH. Critical role of somatosensation in postural control following spaceflight: vestibularly deficient astronauts are not able to maintain upright stance during compromised somatosensation. Front Physiol. 2018;9:1680.
Garrett-Bakelman FE, Darshi M, Green SJ, Gur RC, Lin L, Macias BR, et al. The NASA Twins Study: a multidimensional analysis of a year-long human spaceflight. Science. 2019;364:eaau8650.
Lee JK, Koppelmans V, Riascos RF, Hasan KM, Pasternak O, Mulavara AP, et al. Spaceflight-associated brain white matter microstructural changes and intracranial fluid redistribution. JAMA Neurol. 2019;76:412.
Osterhoff G, Morgan EF, Shefelbine SJ, Karim L, McNamara LM. Bone mechanical properties and changes with osteoporosis. Injury. 2016;47:S11–20.
Reitz G. Characteristic of the radiation field in low Earth orbit and in deep space. Z Med Phys. 2008;18:233–43.
Cosman F, de Beur SJ, LeBoff MS, Lewiecki EM, Tanner B, Randall S, et al. Clinician’s guide to prevention and treatment of osteoporosis. Osteoporos Int. 2014;25:2359–81.
U.S. Preventive Services Task Force. Screening for osteoporosis: U.S. Preventive Services Task Force Recommendation Statement. Ann Intern Med. 2011;154:356.
•• Sibonga JD, Spector ER, Johnston SL, Tarver WJ. Evaluating bone loss in ISS Astronauts. Aerosp Med Hum Perform. 2015;86:38–44 While ISS astronauts show significant reductions in bone mineral density (via DXA measurements), this measurement is not predictive of skeletal fragility. This article proposes new technologies and approaches for evaluating fracture risk in astronauts and points to the need for novel information to guide skeletal assessment.
Shetty S, Kapoor N, Bondu JD, Thomas N, Paul TV. Bone turnover markers: emerging tool in the management of osteoporosis. Indian J Endocrinol Metab. 2016;20:846.
Marshall D, Johnell O, Wedel H. Meta-analysis of how well measures of bone mineral density predict occurrence of osteoporotic fractures. BMJ. 1996;312:1254–9.
Cummings SR, Bates D, Black DM. Clinical use of bone densitometry. JAMA. 2002;288:1889.
Wang X, Shen X, Li X, Mauli Agrawal C. Age-related changes in the collagen network and toughness of bone. Bone. 2002;31:1–7.
Paschalis EP, Mendelsohn R, Boskey AL. Infrared assessment of bone quality: a review. Clin Orthop Relat Res. 2011;469:2170–8 (Springer-Verlag.
Seeman E, Delmas PD. Bone quality—the material and structural basis of bone strength and fragility. N Engl J Med. 2006;354:2250–61.
Hernandez CJ, Keaveny TM. A biomechanical perspective on bone quality. Bone. 2006;39:1173–81.
Bouxsein ML. Bone quality: where do we go from here? Osteoporos Int. 2003;14:118–27.
van der Meulen MC, Jepsen K, Mikić B. Understanding bone strength: size isn’t everything. Bone. 2001;29:101–4.
Boskey AL, Imbert L. Bone quality changes associated with aging and disease: a review. Ann N Y Acad Sci. 2017;1410:93–106.
Donnelly E. Methods for assessing bone quality: a review. Clin Orthop Relat Res. 2011;469:2128–38 (Springer-Verlag.
Roach H. Why does bone matrix contain non-collagenous proteins? The possible roles of osteocalcin, osteonectin, osteopontin and bone sialoprotein in bone mineralisation and resorption. Cell Biol Int. 1994;18:617–28.
Gourion-Arsiquaud S, Faibish D, Myers E, Spevak L, Compston J, Hodsman A, et al. Use of FTIR spectroscopic imaging to identify parameters associated with fragility fracture. J Bone Miner Res. 2009;24:1565–71.
Seeman E. Bone quality: the material and structural basis of bone strength. J Bone Miner Metab. 2008;26:1–8.
Saito M, Marumo K. Collagen cross-links as a determinant of bone quality: a possible explanation for bone fragility in aging, osteoporosis, and diabetes mellitus. Osteoporos Int. 2010;21:195–214.
McCarthy I, Goodship A, Herzog R, Oganov V, Stussi E, Vahlensieck M. Investigation of bone changes in microgravity during long and short duration space flight: comparison of techniques. Eur J Clin Investig. 2000;30:1044–54.
Bembey AK, Bushby AJ, Boyde A, Ferguson VL, Oyen ML. Hydration effects on the micro-mechanical properties of bone. J Mater Res. 2006;21:1962–8.
Nyman JS, Roy A, Shen X, Acuna RL, Tyler JH, Wang X. The influence of water removal on the strength and toughness of cortical bone. J Biomech. 2006;39:931–8.
Zimmermann EA, Ritchie RO. Bone as a structural material. Adv Healthc Mater. 2015;4:1287–304.
Heveran CM, Schurman C, Acevedo C, Livingston EW, Howe D, Schaible EG, et al. Chronic kidney disease and aging differentially diminish bone material and microarchitecture in C57Bl/6 mice. Bone. 2019;127:91–103.
Ural A, Vashishth D. Hierarchical perspective of bone toughness—from molecules to fracture. Int Mater Rev. 2014;59:245–63.
Gordon CL, Lang TF, Augat P, Genant HK. Image-Based Assessment of spinal trabecular bone structure from high-resolution CT images. Osteoporos Int. 1998;8:317–25.
LeBlanc A, Schneider V, Shackelford L, West S, Oganov V, Bakulin A, et al. Bone mineral and lean tissue loss after long duration space flight. J Musculoskelet Neuronal Interact. 2000;1:157–60.
Collet P, Uebelhart D, Vico L, Moro L, Hartmann D, Roth M, et al. Effects of 1- and 6-month spaceflight on bone mass and biochemistry in two humans. Bone. 1997;20:547–51.
Lang T, LeBlanc A, Evans H, Lu Y, Genant H, Yu A. Cortical and trabecular bone mineral loss from the spine and hip in long-duration spaceflight. J Bone Miner Res. 2004;19:1006–12.
Vico L, Hargens A. Skeletal changes during and after spaceflight. Nat Rev Rheumatol. 2018;14:229–45.
Belavý DL, Baecker N, Armbrecht G, Beller G, Buehlmeier J, Frings-Meuthen P, et al. Serum sclerostin and DKK1 in relation to exercise against bone loss in experimental bed rest. J Bone Miner Metab. 2016;34:354–65.
Frings-Meuthen P, Boehme G, Liphardt A-M, Baecker N, Heer M, Rittweger J. Sclerostin and DKK1 levels during 14 and 21 days of bed rest in healthy young men. J Musculoskelet Neuronal Interact. 2013;13:45–52.
Spatz JM, Fields EE, Yu EW, Divieti Pajevic P, Bouxsein ML, Sibonga JD, et al. Serum sclerostin increases in healthy adult men during bed rest. J Clin Endocrinol Metab. 2012;97:E1736–40.
Rittweger J, Debevec T, Frings-Meuthen P, Lau P, Mittag U, Ganse B, et al. On the combined effects of normobaric hypoxia and bed rest upon bone and mineral metabolism: results from the PlanHab study. Bone. 2016;91:130–8.
Vico L, van Rietbergen B, Vilayphiou N, Linossier M-T, Locrelle H, Normand M, et al. Cortical and trabecular bone microstructure did not recover at weight-bearing skeletal sites and progressively deteriorated at non-weight-bearing sites during the year following International Space Station Missions. J Bone Miner Res. 2017;32:2010–21.
Orwoll ES, Adler RA, Amin S, Binkley N, Lewiecki EM, Petak SM, et al. Skeletal health in long-duration astronauts: nature, assessment, and management recommendations from the NASA bone summit. J Bone Miner Res. 2013;28:1243–55.
Leblanc AD, Driscol TB, Shackelford LC, Evans HJ, Rianon NJ, Smith SM, et al. Alendronate as an effective countermeasure to disuse induced bone loss. J Musculoskelet Neuronal Interact. 2002;2:335–43.
LeBlanc A, Matsumoto T, Jones J, Shapiro J, Lang T, Shackelford L, et al. Bisphosphonates as a supplement to exercise to protect bone during long-duration spaceflight. Osteoporos Int. 2013;24:2105–14.
Smith SM, Heer M, Shackelford LC, Sibonga JD, Spatz J, Pietrzyk RA, et al. Bone metabolism and renal stone risk during International Space Station missions. Bone. 2015;81:712–20.
Smith SM, Heer MA, Shackelford LC, Sibonga JD, Ploutz-Snyder L, Zwart SR. Benefits for bone from resistance exercise and nutrition in long-duration spaceflight: evidence from biochemistry and densitometry. J Bone Miner Res. 2012;27:1896–906.
Mashiba T, Hirano T, Turner CH, Forwood MR, Johnston CC, Burr DB. Suppressed bone turnover by bisphosphonates increases microdamage accumulation and reduces some biomechanical properties in dog rib. J Bone Miner Res. 2010;15:613–20.
Sibonga JD, Evans HJ, Sung HG, Spector ER, Lang TF, Oganov VS, et al. Recovery of spaceflight-induced bone loss: bone mineral density after long-duration missions as fitted with an exponential function. Bone. 2007;41:973–8.
Carpenter RD, LeBlanc AD, Evans H, Sibonga JD, Lang TF. Long-term changes in the density and structure of the human hip and spine after long-duration spaceflight. Acta Astronaut. 2010;67:71–81.
Kovacs GTA, Shadden M. Analysis of age as a factor in NASA astronaut selection and career landmarks. PLoS One. 2017;12:e0181381.
Chatani M, Mantoku A, Takeyama K, Abduweli D, Sugamori Y, Aoki K, et al. Microgravity promotes osteoclast activity in medaka fish reared at the international space station. Sci Rep. 2015;5:14172.
Ronca AE, Moyer EL, Talyansky Y, Lowe M, Padmanabhan S, Choi S, et al. Behavior of mice aboard the International Space Station. Sci Rep. 2019;9:4717.
Gridley DS, Nelson GA, Peters LL, Kostenuik PJ, Bateman TA, Morony S, et al. Selected contribution: effects of spaceflight on immunity in the C57BL/6 mouse. II. Activation, cytokines, erythrocytes, and platelets. J Appl Physiol. 2003;94:2095–103.
Pecaut MJ, Nelson GA, Peters LL, Kostenuik PJ, Bateman TA, Morony S, et al. Selected contribution: effects of spaceflight on immunity in the C57BL/6 mouse. I Immune population distributions. J Appl Physiol. 2003;94:2085–94.
• Novoselova EG, Lunin SM, Khrenov MO, Parfenyuk SB, Novoselova TV, Shenkman BS, et al. Changes in immune cell signalling, apoptosis and stress response functions in mice returned from the BION-M1 mission in space. Immunobiology. 2015;220:500–9 This study evaluates stress responses in mice flown in the Bion-M1 mission showing elevated levels of immune parameters that may result from factors (e.g., diet, environment) other than that of microgravity. This study necessitates that future spaceflight studies evaluate for relationships between musculoskeletal changes (e.g, as reported in Gerbaix, Sci. Rep., 2017) and physiological, environmental, and other forms of stress.
Moyer EL, Dumars PM, Sun G-S, Martin KJ, Heathcote DG, Boyle RD, et al. Evaluation of rodent spaceflight in the NASA animal enclosure module for an extended operational period (up to 35 days). NPJ Microgravity. 2016;2:16002.
Liu Y, Biticchi R, Cilli M, Piccardi F, Cancedda R. Mouse Drawer System ( MDS ): an automated payload for supporting rodent research on the international space station. Basic Appl Myol. 2009;19:87–95.
Shimbo M, Kudo T, Hamada M, Jeon H, Imamura Y, Asano K, et al. Ground-based assessment of JAXA mouse habitat cage unit by mouse phenotypic studies. Exp Anim. 2016;65:175–87.
Perlman RL. Mouse models of human disease: an evolutionary perspective. Evol Med Public Health. 2016;2016:170–6.
Beheshti A, McDonald J, Miller J, Grabham P, Costes S. GeneLab database analyses suggest long-term impact of space radiation on the cardiovascular system by the activation of FYN through reactive oxygen species. Int J Mol Sci. 2019;20:661.
Lutwak L, Whedon GD, Lachance PA, Reid JM, Lipscomb HS. Mineral, electrolyte and nitrogen balance studies of the Gemini-VII fourteen-day orbital space flight. J Clin Endocrinol Metab. 1969;29:1140–56.
Smith SM, Mccoy T, Gazda D, Morgan JLL, Heer M, Zwart SR. Space flight calcium: implications for Astronaut Health, Spacecraft Operations, and Earth. Nutrients. 2012;4:2047–68.
Vico L, Chappard D, Palle S, Bakulin AV, Novikov VE, Alexandre C. Trabecular bone remodeling after seven days of weightlessness exposure (BIOCOSMOS 1667). Am J Physiol Integr Comp Physiol. 1988;255:R243–7.
Sibonga J, Zhang M, Evans GL, Westerlind KC, Cavolina JM, Morey-Holton E, et al. Effects of spaceflight and simulated weightlessness on longitudinal bone growth. Bone. 2000;27:535–40.
Wronski TJ, Morey-Holton ER, Doty SB, Maese AC, Walsh CC. Histomorphometric analysis of rat skeleton following spaceflight. Am J Physiol Integr Comp Physiol. 1987;252:R252–5.
Westerlind KC, Turner RT. The skeletal effects of spaceflight in growing rats: tissue-specific alterations in mrna levels for TGF-β. J Bone Miner Res. 2009;10:843–8.
Lloyd SA, Morony SESE, Ferguson VLVL, Simske SJSJ, Stodieck LSLS, Warmington KSKS, et al. Osteoprotegerin is an effective countermeasure for spaceflight-induced bone loss in mice. Bone. 2015;81:562–72.
Backup P, Westerlind K, Harris S, Spelsberg T, Kline B, Turner R. Spaceflight results in reduced mRNA levels for tissue-specific proteins in the musculoskeletal system. Am J Physiol Metab. 2017;266:E567–73.
Vailas AC, Zernicke RF, Grindeland RE, Kaplansky A, Durnova GN, Li KC, et al. Effects of spaceflight on rat humerus geometry, biomechanics, and biochemistry. FASEB J. 1990;4:47–54.
Lafage-Proust MH, Collet P, Dubost JM, Laroche N, Alexandre C, Vico L. Space-related bone mineral redistribution and lack of bone mass recovery after reambulation in young rats. Am J Phys Regul Integr Comp Phys. 1998;274.
Evans GL, Morey-Holton E, Turner RT. Spaceflight has compartment- and gene-specific effects on mRNA levels for bone matrix proteins in rat femur. J Appl Physiol. 1998;84:2132–7.
Bateman TA, Zimmerman RJ, Ayers RA, Ferguson VL, Chapes SK, Simske SJ. Histomorphometric, physical, and mechanical effects of spaceflight and insulin-like growth factor-I on rat long bones. Bone. 1998;23:527–35.
Ferguson VL, Ayers RA, Bateman TA, Simske SJ. Bone development and age-related bone loss in male C57BL/6J mice. Bone. 2003;33:387–98.
Sheng MHC, Baylink DJ, Beamer WG, Donahue LR, Rosen CJ, Lau KHW, et al. Histomorphometric studies show that bone formation and bone mineral apposition rates are greater in C3H/HeJ (high-density) than C57BL/6J (low-density) mice during growth. Bone. 1999;25:421–9.
Silva MJ, Brodt MD, Lynch MA, Stephens AL, Wood DJ, Civitelli R. Tibial loading increases osteogenic gene expression and cortical bone volume in mature and middle-aged mice. PLoS One. 2012;7:e34980.
Beamer WG, Donahue LR, Rosen CJ, Baylink DJ. Genetic variability in adult bone density among inbred strains of mice. Bone. 1996;18:397–403.
Cancedda R, Liu Y, Ruggiu A, Tavella S, Biticchi R, Santucci D, et al. The mice drawer system (MDS) experiment and the space endurance record-breaking mice. PLoS One. 2012;7:e32243.
Tavella S, Ruggiu A, Giuliani A, Brun F, Canciani B, Manescu A, et al. Bone turnover in wild type and pleiotrophin-transgenic mice housed for three months in the international space station (ISS). PLoS One. 2012;7:e33179.
Dadwal UC, Maupin KA, Zamarioli A, Tucker A, Harris JS, Fischer JP, et al. The effects of spaceflight and fracture healing on distant skeletal sites. Sci Rep. 2019;9:11419.
Blaber EA, Dvorochkin N, Lee C, Alwood JS, Yousuf R, Pianetta P, et al. Microgravity induces pelvic bone loss through osteoclastic activity, osteocytic osteolysis, and osteoblastic cell cycle inhibition by CDKN1a/p21. PLoS One. 2013;8:e61372.
Macaulay TR, Siamwala JH, Hargens AR, Macias BR. Thirty days of spaceflight does not alter murine calvariae structure despite increased Sost expression. Bone Rep. 2017;7:57–62.
• Blaber EA, Dvorochkin N, Torres ML, Yousuf R, Burns BP, Globus RK, et al. Mechanical unloading of bone in microgravity reduces mesenchymal and hematopoietic stem cell-mediated tissue regeneration. Stem Cell Res. 2014;13:181–201 Bone changes were observed in group-housed mice flown in NASA rodent habitats, where osteocytes, along with osteoclasts, are implicated in reduced bone quantity and structural quality.
Keune JA, Philbrick KA, Branscum AJ, Iwaniec UT, Turner RT. Spaceflight-induced vertebral bone loss in ovariectomized rats is associated with increased bone marrow adiposity and no change in bone formation. NPJ Microgravity. 2016;2:16016.
Zhang C, Li L, Jiang Y, Wang C, Geng B, Wang Y, et al. Space microgravity drives transdifferentiation of human bone marrow–derived mesenchymal stem cells from osteogenesis to adipogenesis. FASEB J. 2018;32:4444–58.
Nabavi N, Khandani A, Camirand A, Harrison RE. Effects of microgravity on osteoclast bone resorption and osteoblast cytoskeletal organization and adhesion. Bone. 2011;49:965–74.
Kumei Y, Shimokawa H, Ohya K, Katano H, Akiyama H, Hirano M, et al. Small GTPase Ras and Rho expression in rat osteoblasts during spaceflight. Ann N Y Acad Sci. 2007;1095:292–9.
Kumei Y, Morita S, Katano H, Akiyama H, Hirano M, Oyha K, et al. Microgravity signal ensnarls cell adhesion, cytoskeleton, and matrix proteins of rat osteoblasts: osteopontin, CD44, osteonectin, and α-tubulin. in. Ann N Y Acad Sci. 2006;1090:311–7.
Schiller HB, Fässler R. Mechanosensitivity and compositional dynamics of cell–matrix adhesions. EMBO Rep. 2013;14:509–19.
Guignandon A, Faure C, Neutelings T, Rattner A, Mineur P, Linossier M-T, et al. Rac1 GTPase silencing counteracts microgravity-induced effects on osteoblastic cells. FASEB J. 2014;28:4077–87.
Meyers VE, Zayzafoon M, Douglas JT, McDonald JM. RhoA and cytoskeletal disruption mediate reduced osteoblastogenesis and enhanced adipogenesis of human mesenchymal stem cells in modeled microgravity. J Bone Miner Res. 2005;20:1858–66.
Sun Z, Cao X, Zhang Z, Hu Z, Zhang L, Wang H, et al. Simulated microgravity inhibits L-type calcium channel currents partially by the up-regulation of miR-103 in MC3T3-E1 osteoblasts. Sci Rep. 2015;5:8077.
Yan M, Wang Y, Yang M, Liu Y, Qu B, Ye Z, et al. The effects and mechanisms of clinorotation on proliferation and differentiation in bone marrow mesenchymal stem cells. Biochem Biophys Res Commun. 2015;460:327–32.
Dai Z, Wu F, Chen J, Xu H, Wang H, Guo F, et al. Actin microfilament mediates osteoblast Cbfa1 responsiveness to BMP2 under simulated microgravity. PLoS One. 2013;8:e63661.
Kapitonova MY, Kuznetsov SL, Salim N, Othman S, Kamauzaman TMHTM, Ali AM, et al. Morphological and phenotypical characteristics of human osteoblasts after short-term space mission. Bull Exp Biol Med. 2014;156:393–8.
Rea G, Cristofaro F, Pani G, Pascucci B, Ghuge SA, Corsetto PA, et al. Microgravity-driven remodeling of the proteome reveals insights into molecular mechanisms and signal networks involved in response to the space flight environment. J Proteome. 2016;137:3–18.
Sambandam Y, Townsend MT, Pierce JJ, Lipman CM, Haque A, Bateman TA, et al. Microgravity control of autophagy modulates osteoclastogenesis. Bone. 2014;61:125–31.
• Gerbaix M, Gnyubkin V, Farlay D, Olivier C, Ammann P, Courbon G, et al. One-month spaceflight compromises the bone microstructure, tissue-level mechanical properties, osteocyte survival and lacunae volume in mature mice skeletons. Sci Rep. 2017;7:2659 Bone changes were observed in group-housed mice flown in the Bion-M1 rodent habitat, where osteocytes are implicated in reduced bone tissue-level mechanical properties. along with reduced populations of mesenchymal and hematopoietic stem cells and cell-mediated tissue regeneration. No changes in bone biochemistry were observed.
Cabahug-Zuckerman P, Frikha-Benayed D, Majeska RJ, Tuthill A, Yakar S, Judex S, et al. Osteocyte apoptosis caused by hindlimb unloading is required to trigger osteocyte RANKL production and subsequent resorption of cortical and trabecular bone in mice femurs. J Bone Miner Res. 2016;31:1356–65.
Glatt V, Canalis E, Stadmeyer L, Bouxsein ML. Age-related changes in trabecular architecture differ in female and male C57BL/6J mice. J Bone Miner Res. 2007;22:1197–207 (John Wiley & Sons, Ltd.
Coulombe JC, Livingston EW, Ortega AM, Bateman TA, Vance EA, Stodieck LS, et al. Microgravity exposure diminishes trabecular microarchitecture and cortical bone structure differently in growing and skeletally mature mice (Conference Presentation). J Bone Miner Res. 2018;33:326.
Bonewald LF. The amazing osteocyte. J Bone Miner Res. 2011;26:229–38.
Qing H, Ardeshirpour L, Divieti Pajevic P, Dusevich V, Jähn K, Kato S, et al. Demonstration of osteocytic perilacunar/canalicular remodeling in mice during lactation. J Bone Miner Res. 2012;27:1018–29.
Rodionova NV, Oganov VS, Zolotova NV. Ultrastructural changes in osteocytes in microgravity conditions. Adv Space Res. 2002;30:765–70.
Mader KS, Schneider P, Müller R, Stampanoni M. A quantitative framework for the 3D characterization of the osteocyte lacunar system. Bone. 2013;57:142–54.
Heveran CM, Rauff A, King KB, Carpenter RD, Ferguson VL. A new open-source tool for measuring 3D osteocyte lacunar geometries from confocal laser scanning microscopy reveals age-related changes to lacunar size and shape in cortical mouse bone. Bone. 2018;110:115–27.
Coulombe JC, Mullen ZK, Wiens AM, Stodieck LS, Ferguson VL. Microgravity exposure in growing mice is detrimental to osteocyte lacunar volume and shape (Conference Presentation). J Bone Miner Res. 2018;33:98.
Pavalko FM, Norvell SM, Burr DB, Turner CH, Duncan RL, Bidwell JP. A Model for mechanotransduction in bone cells: the load-bearing mechanosomes. J Cell Biochem. 2003;88:104–12.
Zernicke RF, Vailas AC, Grindeland RE, Kaplansky A, Salem GJ, Martinez DA. Spaceflight effects on biomechanical and biochemical properties of rat vertebrae. Am J Physiol Integr Comp Physiol. 1990;258:R1327–32.
Simmons DJ, Grynpas MD, Rosenberg GD. Maturation of bone and dentin matrices in rats flown on the Soviet biosatellite Cosmos 1887. FASEB J. 1990;4:29–33.
Simmons DJ, Russell JE, Grynpas MD. Bone maturation and quality of bone material in rats flown on the space shuttle ‘Spacelab-3 Mission’. Bone Miner. 1986;1:485–93.
Patterson-Buckendahl P, Arnaud SB, Mechanic GL, Martin RB, Grindeland RE, Cann CE. Fragility and composition of growing rat bone after one week in spaceflight. Am. J. Physiol. Integr. Comp. Physiol. 1987;252:R240–6.
Mechanic GL, Arnaud SB, Boyde A, Bromage TG, Buckendahl P, Elliott JC, et al. Regional distribution of mineral and matrix in the femurs of rats flown on Cosmos 1887 biosatellite. FASEB J. 1990;4:34–40.
France EP, Oloff CM, Kazarian LE. Bone Mineral Analysis of Rat Vertebra Following Spaceflight: Cosmos 1129. Physiologist, supl. 1983;25(6):S147-S148.
Simmons DJ, Russell JE, Winter F, Tran Van P, Vignery A, Baron R, et al. Effect of spaceflight on the non-weight-bearing bones of rat skeleton. Am. J. Physiol. Integr. Comp. Physiol. 1983;244:R319–26.
Glimcher MJ. The nature of the mineral component of bone and the mechanism of calcification. Instr Course Lect. 1987;36:49–69.
Shaw SR, Vailas AC, Grindeland RE, Zernicke RF. Effects of a 1-wk spaceflight on morphological and mechanical properties of growing bone. Am. J. Physiol. Integr. Comp. Physiol. 1988;254:R78–83.
Vernikos J, Schneider VS. Space, gravity and the physiology of aging: parallel or convergent disciplines? A mini-review. Gerontology. 2010;56:157–66.
Liu Y, Wang E. Transcriptional analysis of normal human fibroblast responses to microgravity stress. Genomics Proteomics Bioinformatics. 2008;6:29–41.
Radugina EA, Almeida EAC, Blaber E, Poplinskaya VA, Markitantova YV, Grigoryan EN. Exposure to microgravity for 30 days onboard Bion M1 caused muscle atrophy and impaired regeneration in murine femoral Quadriceps. Life Sci Space Res. 2018;16:18–25.
Jonscher KR, Alfonso-Garcia A, Suhalim JL, Orlicky DJ, Potma EO, Ferguson VL, et al. Spaceflight activates lipotoxic pathways in mouse liver. PLoS One. 2016;11.
Mao XW, Nishiyama NC, Byrum SD, Stanbouly S, Jones T, Drew A, et al. Characterization of mouse ocular response to a 35-day spaceflight mission: evidence of blood-retinal barrier disruption and ocular adaptations. Sci Rep. 2019;9:8215.
Mao XW, Pecaut MJ, Stodieck LS, Ferguson VL, Bateman TA, Bouxsein M, et al. Spaceflight environment induces mitochondrial oxidative damage in ocular tissue. Radiat Res. 2013;180.
Mao X, Sandberg L, Gridley D, Herrmann E, Zhang G, Raghavan R, et al. Proteomic analysis of mouse brain subjected to spaceflight. Int J Mol Sci. 2018;20:7.
Lang T, Van Loon JJWA, Bloomfield S, Vico L, Chopard A, Rittweger J, et al. Towards human exploration of space: the THESEUS review series on muscle and bone research priorities. NPJ Microgravity. 2017;3:8.
Gridley DS, Mao XW, Stodieck LS, Ferguson VL, Bateman TA, Moldovan M, et al. Changes in mouse thymus and spleen after return from the STS-135 mission in space. PLoS One. 2013;8:e75097.
Sofronova SI, Tarasova OS, Gaynullina D, Borzykh AA, Behnke BJ, Stabley JN, et al. Spaceflight on the Bion-M1 biosatellite alters cerebral artery vasomotor and mechanical properties in mice. J Appl Physiol. 2015;118:830–8.
Taylor CR, Hanna M, Behnke BJ, Stabley JN, McCullough DJ, Davis RT, et al. Spaceflight-induced alterations in cerebral artery vasoconstrictor, mechanical, and structural properties: implications for elevated cerebral perfusion and intracranial pressure. FASEB J. 2013;27:2282–92.
Schaffler MB, Choi K, Milgrom C. Aging and matrix microdamage accumulation in human compact bone. Bone. 1995;17:521–5.
Riggs BL, Melton LJ, Robb RA, Camp JJ, Atkinson EJ, Peterson JM, et al. Population-based study of age and sex differences in bone volumetric density, size, geometry, and structure at different skeletal sites. J Bone Miner Res. 2004;19:1945–54.
Russo CR, Lauretani F, Seeman E, Bartali B, Bandinelli S, Di Iorio A, et al. Structural adaptations to bone loss in aging men and women. Bone. 2006;38:112–8.
Vashishth D, Verborgt O, Divine G, Schaffler MB, Fyhrie DP. Decline in osteocyte lacunar density in human cortical bone is associated with accumulation of microcracks with age. Bone. 2000;26:375–80.
Akkus O, Polyakova-Akkus A, Adar F, Schaffler MB. Aging of microstructural compartments in human compact bone. J Bone Miner Res. 2003;18:1012–9.
Burr DB. Changes in bone matrix properties with aging. Bone. 2019;120:85–93.
Cavolina JM, Evans GL, Harris SA, Zhang M, Westerlind KC, Turner RT. The effects of orbital space flight on bone histomorphometry and messenger ribonucleic acid levels for bone matrix proteins and skeletal signaling peptides in ovariectomized growing rats. Endocrinology. 1997;138:1567–76.
Petit MA, Beck TJ, Lin H-M, Bentley C, Legro RS, Lloyd T. Femoral bone structural geometry adapts to mechanical loading and is influenced by sex steroids: the Penn State Young Women’s Health Study. Bone. 2004;35:750–9.
Mahoney E. Sending American Astronauts to Moon in 2024: NASA Accepts Challenge. NASA Press Release. 2019.
Schierholz S, Jorgan G, Northon K. (Editor). NASA Opens International Space Station to New Commercial Opportunities. NASA Press Release. 2019.
Acknowledgments
Funding was provided by NSBRI MA00002, BL01302; NASA NNJ10GA25A, NNX10AE39G-S1. Thank you to Louis Stodieck, BioServe Space Technologies, University of Colorado at Boulder, for helpful input.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Jennifer Coulombe, Bhavya Senwar, and Virginia L. Ferguson declare no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Biomechanics
Electronic supplementary material
ESM 1
(DOCX 30 kb)
Rights and permissions
About this article
Cite this article
Coulombe, J., Senwar, B. & Ferguson, V.L. Spaceflight-Induced Bone Tissue Changes that Affect Bone Quality and Increase Fracture Risk. Curr Osteoporos Rep 18, 1–12 (2020). https://doi.org/10.1007/s11914-019-00540-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11914-019-00540-y