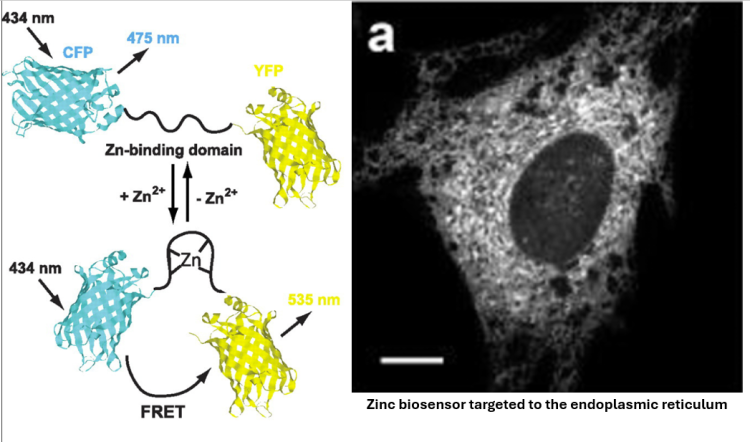
Illuminating the role of zinc in cell biology
Zinc is the second most abundant transition metal in biology and plays a crucial role in a wide variety of cellular processes. Roughly 10% of the human genome encodes for zinc-binding proteins and all cells must regulate and maintain their own pool of labile zinc. It has been shown that zinc is essential for cell growth and proliferation as a biological cofactor and as a signaling ion. It also plays a role in genome integrity, immune function, and the senses of taste and smell! Despite its critical role in cell physiology and human health, many questions remain regarding its mechanisms of action. The Palmer Lab uses novel fluorescent metal ion sensors and in vitro mammalian cell experiments to investigate zinc dynamics in different contexts. For example, we use multiple types of breast cancer cells to study the involvement of zinc in cancer and tumor growth, and primary hippocampal neurons to study the role of zinc in the brain and nervous system.