CU Boulder chemistry researcher Joel Eaves and his co-investigators demonstrate how designing interfaces between organic and inorganic materials can convert low-energy light to high-energy
A new class of materials made from inorganic silicon nanoparticles and a common hydrocarbon molecule has the potential to not only make solar panels more efficient, but to improve certain medical imaging and even enhance night vision goggles.
These materials, created by a National Science Foundation-funded group of researchers including Joel Eaves, a professor in the University of Colorado Boulder Department of Chemistry, are detailed in research newly published in Nature Chemistry.
Joining inorganic and organic components is an area of chemistry that hasn’t been widely explored but shows great promise in addressing the need to move photon energies around, Eaves explains.

Theoretical chemist Joel Eaves partners with colleagues around the country on research exploring nanotechnology and chemical physics.
“The synthetic ability to design these interfaces between organic molecules and inorganic nanoparticles is so enabling,” Eaves says. “There are a lot of really fundamental things we’d like to do and I don’t know what those big next steps are yet, but the process of turning photons into other photons and photons into chemical fuels is something people have been working on for decades. These new materials may lead us to places we’ve yet to reach in these areas.”
Strengthening electronic bonds
Eaves and his colleagues worked with nanometer-sized silicon particles, also called quantum dots, and anthracene, a type of hydrocarbon often used in producing certain dyes. Typically, the electronic bond between the organic molecules and inorganic silicon dots is weak.
Due to this weak bonding, energy moves across the interface in incoherent hops. Eaves and his research colleagues strengthened the bond between the two dissimilar components to create a single new material whose electronic properties were hybridized and distinct from those of its individual components. This significantly improved the efficiency with which the molecules can convert lower-energy red light to higher-energy blue light—a process called photon up-conversion.
The implications and potential applications of this new material may be far-reaching, Eaves says. For example, it may increase solar cell efficiencies by manipulating the spectrum of light.
Eventually, there also may be potential to enhance light-based medical imaging and treatment. While near-infrared light can penetrate deeply into the body, it doesn’t have the energy to generate the free radicals that high-energy ultraviolet light can. Some cancer treatment uses these ultraviolet-generated free radicals to attack cancer tissue, so there is potential to send energy to a targeted spot in the body up-converting red photons so that they damage and kill cancer cells.
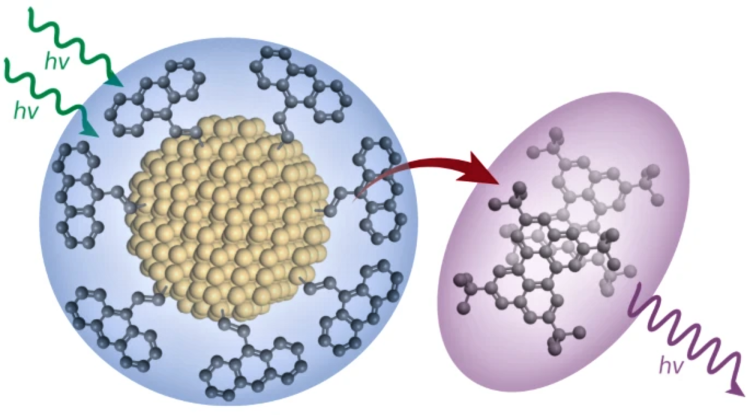
Research shows that strengthening the bond between inorganic silicon nanoparticles and a common hydrocarbon molecule creates a new, more energy-efficient material.
Building capacity in research
Another exciting aspect of this new material is its use of silicon, which is earth-abundant and non-toxic, unlike more conventional nanoparticles that use toxic materials like cadmium or lead—making them unusable for medical applications.
While the silicon dots used in the research were created by co-researcher Lorenzo Mangolini, who leads one of just a handful of labs currently able to make them, “as the barrier to entry becomes lower on these next-level technologies, people figure out how to do things better and we train more people to make these components,” Eaves says. “Over time, there will be more people with the ability and capacity to make these things and more innovation will result.”
Eaves adds that beyond the thrill of tremendous new doors that this research opens is the fundamental joy of scientific discovery: “I’m excited about the fact that we have a new chemistry, we have a new material and that we’ve demonstrated how device performance improves when strengthening one chemical bond. The capability is really exciting, not just for next steps in research but what it means for chemistry.”
Former CU Boulder researcher R. Peyton Cline, now at NREL in Golden, Colorado; Kefu Wang and Ming Lee Tang of the University of Utah; Joseph Schwan and Lorenzo Mangolini of University of California Riverside; and Sean T. Roberts and Jacob M. Strain of the University of Texas Austin also contributed extensively to the research.



